Packaging,Traceability and Autoclave Control Tests
Packaging, Traceability and Autoclave Control Tests
These products are designed for hospital, medical, dental, cosmetic, veterinary and podology uses and, in general, for all operators involved in steam or gas sterilization processes. The T paper products are made of composite plastic material (polyester-polypropylene) and special medical paper, moisture resistant, and with high anti-bacterial protection.
The bag sealing system prevents air bubble formation and ensures a perfect seal, avoiding fine dust penetration, which would result in non-sterile conditions.
Water-based inks are used. The rolls have a toning indicator at every 10 cm, while each bag has one toning indicator.
Description
Packaging,Traceability and Autoclave Control Tests
A.Packaging
1.One-Thermosealer

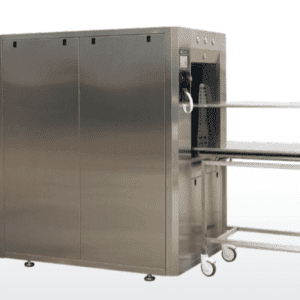
One: ONE is a new generation sealer that has been created to reduce envelope packaging time, monitor the welding quality, and seal all types of paper for sterilization, including folded paper.

Technical Features

2.Tecno Seal Print
ROLL THERMOSEALER WITH INTEGRATED PRINTER AND AUTOMATIC PHOTOCELL FEED SYSTEM
TECNO SEAL PRINT: Tecno Seal Print is a roll thermosealer with integrated printer and automatic photocell feed system. This is the ultimate expression of technology applied to medical sealing, used in medical centres, dental surgeries, hospitals and clinics. It guarantees very high output, maximum operating speed without routine maintenance; it uses precut bags sealed on 3 sides (available in various sizes and supplied by Tecno-Gaz). Tecno Seal Print has an integrated printer which, during sealing, automatically prints on the bag the packaging and expiry date, as well as the required regulatory symbols. This important feature allows constant control over the current sterile conditions of the instruments. Another feature is an electronic control and management card allowing the use parameter adjustments. The use of this machine is simple and rational. The operator only has to insert the bag containing the instrument into the sealing machine support; the photocell automatically, autonomously feeds the bag, seals it and prints the packaging data.This action can be continuative and repetitive to ensure high output and considerable time reduction.Surgeries which intend to certify and document all the sterilization procedures must have Tecno Seal Print.
Technical Features

3.Tecno Seal
ROLL THERMOSEALER WITH AUTOMATIC PHOTOCELL FEED SYSTEM

Tecno Seal is a roll thermosealer with automatic photocell feed system. This is the ultimate expression of technology applied to medical sealing, used in medical centres, dental surgeries, hospitals and clinics. It guarantees very high output, maximum operating speed without routine maintenance; it uses precut bags sealed on 3 sides (available in various sizes and supplied by Tecno-Gaz). This important feature allows constant control over the current sterile conditions of the instruments. Another feature is an electronic control and management card allowing the use parameter adjustments. The use of this machine is simple and rational. The operator only has to insert the bag containing the instrument into the sealing machine support; the photocell automatically, autonomously feeds the bag and seals it.
For more information please click here to watch the video.
4.T Paper


These products are designed for hospital, medical, dental, cosmetic, veterinary and podology uses and, in general, for all operators involved in steam or gas sterilisation processes.The T paper products are made of composite plastic material (polyester-polypropylene) and special medical paper, moisture resistant and with high anti-bacteria protection.The bag sealing system prevents air bubble formation and ensures a perfect seal, avoiding fine dust penetration, which would result in non sterile conditions.Water-based inks are used. The rolls have a toning indicator at every 10 cm, while each bag has one toning indicator.The indicators must be used for steam or gas toning.
B.Traceability
1.Traccia

Traccia: Traccia Instrument tracking is a basic procedure for the handling of sterilized materials. Tracking guarantees the recording of the course of each instrument, from preparation to sterilization, until its use. This is possible because of a cross checking data management system. Tracking forms an integral part of the sterilisation cycle procedure and should be performed after the wrapping phase and before autoclave loading. This procedure is indispensable to guarantee maximum legal protection to both operators and health director.
Technical Features


2.Tecno Print
PRINTER FOR BAR CODE TRACKING, FOR EUROPA B PRO, ONYX

Printer for bar code tracking, for Europa B pro, Alia and Aura
C.Autoclave control tests
1.B-Test Plus
Biological incubator independently powered
 B-Test PLUS Independently powered 230 vac
B-Test PLUS Independently powered 230 vac
Our biological incubator is a product developed for the incubation of biological indicators at a temperature of 57°C.
The incubator consists in a frame, an ABS cover, an aluminium heating unit with 3 slots for test tubes/biological indicators and an electronic card for automatic control of heating unit temperature.
B-TEST PLUS
The Plus version is equipped with a cable for power mains connection (230 vac absorption).
Technical Features


2.Bowie & Dick Test
Sterilisation Test, 20 pcs

Bowie e Dick Test Control test to measure the ability to remove air pockets from porous items. Only suitable for class B autoclaves.
What are these used for?
Physical test, only suitable for autoclaves that are able to sterilize porous loads. It is used to asses the ability of steam penetration in porous bodies.Requires the use of spores in phials and a biological incubator.Frequency Recommended every 30 days.
Technical Features

3.Helix Test
Sterilisation Test

Control test to measure the ability to remove air pockets from hollow items. Only suitable for class B autoclaves.
What are these used for?
It is used to assess the ability of steam penetration in hollow bodies.
Frequency Recommended every 30 days.
Technical Features

4.SteamPlus
Sterilisation Test

SteamPlus BIOLOGICAL INTEGRATORS (100 pcs) Chemical tests for the validation of sterilisation cycle. Suitable for any type of autoclave
5.Vapor Line
Sterilisation Test

VAPORLINE BIOLOGICAL INTEGRATORS (250 pcs) Chemical tests for the validation of sterilisation cycle. Suitable for any type of autoclave.
What are these used for?
Tests suitable for all types of autoclaves. They are used to assess the ability of colour change in the autoclaves through the parameters of the machine time temperature – pressure.
Frequency Recommended once a day.
6.Sterildocs
Autoclave test filing system

Tecno-Gaz S.p.A. has developed a new filing system called STERILDOCS.Every owner of a medical device must periodically check that it is in working order according to procedures defined by the manufacturer, current standards or directives. For autoclaves, there are specific tests and control systems laid down by directives, regional laws and regulations. The tests certify that the equipment is valid and in working order and must be filed and, preferably, kept for 10 years of owner’s liability.
STERILDOCS is a file with the following sections:
– Support section operating protocol DVDs.
– Written protocol instruction section including sterilisation tests.
– Surgery and equipment data sheet section.
– Recorded practice and autoclave data section. The paper used is watermarked, to prevent modifications or corrections.
– Filing section divided into sectors and identified by a specific graphical structure which enables user-friendly filing of each test, providing information on data, cycles, etc.
Filing is simple, rational and tidy, and allows organic management of the tests of each autoclave.
7.Spores
Sterilisation Test

SPORE VIAL kit including 20 pcs of spores for biological control. Suitable for any type of autoclave.
For more information don’t hesitate to contact us here!
.
Additional information
| Item Condition | |
|---|---|
| Manufacturer |